One of the great challenges in medicine is in weighing the potential benefits of therapeutic strategies against their potential risks. In some cases, the decision may be fairly clear – chemotherapeutic drugs may wreak havoc on the body, but when the alternative is dying of cancer, most physicians and patients opt for treatment despite the side effects, at least when there is a clear survival advantage. But quite often, the risks and benefits are more nuanced or less certain, and the choice as to whether or not to proceed with a given therapy is far more complex. Indeed, one of the areas in which these decisions are perhaps most fraught is in the pharmacological treatment of certain psychiatric disorders such as attention deficit hyperactivity disorder (ADHD).
ADHD, though commonly diagnosed during childhood and historically thought to resolve by adulthood, is now widely recognized as a lifelong disorder characterized by impulsivity, inattentiveness, hyperactivity, emotional dysregulation, and other symptoms of executive dysfunction. Currently estimated to affect 5-10% of US children and adults (though prevalence is on the rise), ADHD is typically treated with various stimulant (and to a lesser extent, non-stimulant) medications – which many patients take for decades following diagnosis. Thus, there is a clear need to understand the long-term health implications of these therapies – a need which motivated investigators Zhang et al. to conduct a recent case-control study on the association between ADHD medications and cardiovascular disease (CVD).1
What they did
Zhang et al. conducted a nested case-control study using data from various Swedish national health registers. (In contrast to classical case-control studies, in nested case-control studies, cases and controls are all sampled from the same predefined source cohort of known size – in this case, individuals in the Swedish national health register.) All individuals aged 6 to 64 years in Sweden who received an incident diagnosis of ADHD or a dispensation for ADHD medication between 2007 and 2020 (n=278,027) were included, with cases defined as those who went on to develop CVD (including ischemic heart diseases, cerebrovascular diseases, hypertension, heart failure, arrhythmias, thromboembolic disease, arterial disease, and other forms of heart disease) in the follow-up period after their ADHD diagnosis. Each case was matched for age, sex, and calendar time (to ensure the same duration of follow-up) with up to five controls (i.e., individuals who did not develop CVD over the follow-up period). (Note that case-control studies often use control-to-case ratios greater than one in order to increase statistical power.)
The date of incident ADHD diagnosis or ADHD medication dispensation (whichever came first) served as the baseline date for each participant, and the index date was defined as the date of CVD diagnosis (cases) or, for controls, the date of CVD diagnosis for the matched case. Individuals who had CVD at baseline or received ADHD medication for indications other than ADHD (e.g., narcolepsy, depression) were excluded, as were any individuals with a follow-up period of less than three months. Ultimately, this resulted in a final analysis cohort of 10,388 cases (median age at baseline: 34.6 years; 59.2% male) and 51,672 age- and sex-matched controls.
What they found
Median follow-up time was 4.1 years. The majority of both cases (83.9%) and controls (83.5%) were reported to have used ADHD medications during the follow-up period. The most commonly used medication was methylphenidate (a stimulant drug known by the trade names Ritalin, Concerta, and others), followed by atomexine (a selective norepinephrine reuptake inhibitor often taken in combination with stimulant drugs, known by the trade name Strattera) and lisdexamfetamine (a stimulant composed of dextroamphetamine bound to L-lysine, known by the trade names Vyvanse and Elvanse). Of note, unbound dextroamphetamine (trade names Adderall, Dexedrine, and others) is one of the most commonly used medications for ADHD in the US but is not approved for this purpose in Europe; thus, this compound is not represented in this Sweden-based study.
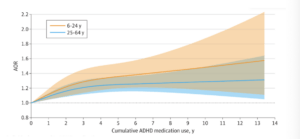
Across the full cohort, the incidence rate of any type of CVD was 7.34 per 1000 person-years, with the most common type being hypertension (4,210 cases; 40.5%), followed by arrhythmias (1,310 cases; 12.6%). After adjusting for covariates (country of birth, educational level, type 2 diabetes, obesity, dyslipidemia, sleep disorders, and psychiatric comorbidities), the authors reported a positive association between ADHD medication use and CVD risk, with longer durations of medication use associated with increasingly elevated risk up to 3-5 years of cumulative use, which corresponded to a 27% increase in risk relative to non-use (adjusted odds ratio: 1.27; 95% CI: 1.17-1.39). Beyond five years of use, excess risk appeared to stabilize (Figure 1). Higher daily doses were also found to be associated with marginally higher (4-5%) CVD risk, but only at doses at least 1.5x higher than the average daily dose of 30 mg. Similar results were observed when examining females and males separately, whereas separate analyses of participants under 25 years of age versus 25 and over indicated that longer durations of medication use may impart greater elevations in CVD risk among children and adolescents than among adults (Figure 2).
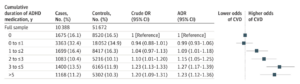
In sub-analyses for specific categories of CVD, Zhang et al. reported that use of ADHD medications for at least three years correlated with a significant increases in risk of hypertension (AOR: 1.72; 95% CI: 1.51-1.97 for 3 to ≤5 years of use; AOR: 1.80; 95% CI: 1.55-2.08 for >5 years) and in arterial disease (AOR: 1.65; 95% CI, 1.11-2.45 for 3 to ≤5 years; AOR: 1.49; 95% CI: 0.96-2.32 for >5 years). (Note: arterial disease was defined as ICD-9 codes 440-444, which include atherosclerosis, aneurysms of the aorta or other vessels, aortic dissection, aortic embolism or thrombosis, or “other peripheral vascular disease” – a broad term, to say the least.) No statistically significant risk increases were observed for arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease, or for hypertension or arterial disease following short-term (<3 years) use of medication.
Sub-analyses by medication type revealed that long-term use of methylphenidate (e.g., Ritalin) was associated with an increased risk of CVD relative to no use (AOR: 1.20; 95%CI: 1.10-1.31 for 3 to ≤5 years; AOR: 1.19; 95% CI: 1.08-1.31 for >5 years). Lisdexamfetamine was also associated with an elevated risk of CVD (AOR: 1.23; 95% CI: 1.05-1.44 for 2 to ≤3 years; AOR: 1.17; 95% CI: 0.98-1.40 for >3 years). Atomoxetine use was associated with a significant increase in CVD risk only for the first year of use (AOR: 1.07; 95% CI: 1.01-1.13).
Discrepancies in baseline risk
Based on these findings, the authors of this study conclude that long-term use of ADHD medications, particularly stimulant medications, are associated with increased CVD risk, specifically in the form of hypertension and arterial disease. But as with any case-control study, this conclusion depends critically on how well the groups are matched at baseline.
Previous research has indicated that unmanaged ADHD is associated with higher rates of substance abuse, obesity, anxiety & stress disorders, sleep disorders, and other major risk factors for CVD.2–4 (Mechanistically, the idea that some or all of these associations represent causal relationships is highly likely.) Thus, in this study, both CVD cases and controls had ADHD diagnoses, and the exposure of interest was ADHD medication use, not presence/absence of ADHD. This is a critical strength of the study, and with this design, the results imply that use of ADHD medications is associated with an increase in CVD risk that exceeds any risk associated with unmanaged ADHD.
However, despite cases and controls being matched for age, sex, and ADHD diagnosis, baseline characteristics reveal significant differences between the two groups, with cases exhibiting higher rates of comorbidities associated with CVD risk, including obesity, type 2 diabetes, sleep disorders, substance abuse, and anxiety/psychiatric disorders. For instance, the rate of substance use disorders among cases was over 30% higher among cases than among controls, while cases were also 60% more likely to have obesity and more than twice as likely to have type 2 diabetes. Zhang et al. adjusted for these specific covariates, but these discrepancies collectively imply that cases had poorer health overall than controls, which could easily extend to variables that were not included in adjustments, such as exercise habits and use of other medications. Further, binary adjustment for the presence versus absence of obesity, substance use, or other covariates doesn’t take into account that such variables exist on a spectrum – a BMI of 20 or a BMI of 29 would both be treated simply as an absence of obesity, despite the former falling within a healthy BMI range and the latter categorized as overweight.
Any one of the discrepancies in comorbidity rates might be enough to call into question the study conclusions, but together, they follow a pattern strikingly similar to that mentioned above regarding unmanaged ADHD, which might suggest that cases suffered from more severe ADHD on average than controls. Pharmacological treatment has been shown to reduce the risk or severity of some – but not all – comorbidities associated with unmanaged ADHD.2 Thus, greater severity of ADHD (which would likely correlate with longer medication use) might be expected to increase risk of CVD independently of medication. The fact that individuals under age 25 evidently experience a greater elevation in risk with cumulative medication use than those over age 25 would agree with this alternative explanation, as the behavioral features of ADHD are more pronounced in more severe cases and are likely to result in diagnosis at an earlier age. (Indeed, the relative difference in comorbidity rates between cases and controls tended to be larger among those under 25 than among those 25 and older).
Weighing cardiovascular risk against other benefits
A question that this study was not designed to evaluate – but which nevertheless is central to this discussion – is how the potential elevation in cardiovascular risk weighs against the quality of life benefits that many ADHD patients derive from pharmacological treatments, as well as the reported benefits in mortality risk more broadly.
The authors reported a significant increase in risk only in hypertension and arterial disease following at least three years of medication use, whereas no correlations were observed with short-term medication use or between long-term medication and other forms of CVD. Further, it’s worth noting that although “arterial disease” includes atherosclerosis and other serious heart conditions, it also includes common but relatively harmless conditions such as primary Raynaud’s syndrome,5 which is a well-documented side effect of stimulants used for ADHD6 and thus likely accounted for many if not most of the cases classified as “arterial disease.” In other words, it’s unclear to what extent the finding on arterial disease has real clinical relevance.
Still, given the high population-wide prevalence of hypertension in most developed countries, the absolute increase in risk associated with ADHD medication is certainly substantial. As we’ve discussed in detail on The Drive, high blood pressure has devastating effects on long-term health, so for those who are already at high risk of hypertension or CVD, this added risk associated with ADHD medications may offset any potential benefits, particularly if ADHD is less severe and might be managed adequately with behavioral therapy as an alternative. Conversely, for those at low baseline risk of CVD, the additional risk of hypertension associated with ADHD medications may be a worthwhile tradeoff for the reported benefits – which range from improved social function and self-esteem to lower rates of driving accidents to fewer addictive behaviors (all of which may contribute to observations of shorter lifespan among those with untreated versus treated ADHD).7
The bottom line
When evaluating the relative risks and benefits of any therapy, more information generally translates to better decisions. Thus, I applaud Zhang et al.’s attempt to add to the collective pool of data on ADHD medications by investigating their associations with cardiovascular health, but we must keep in mind that any single piece of information can be misleading if we fail to place it in a larger context.
First, let’s be clear: the stimulant medications most commonly prescribed for ADHD management increase blood pressure, potentially to a level that meets clinical criteria for hypertension, which may in turn increase risk of other aspects of CVD. This was a well-documented side effect of these drugs long before this study. For individuals who do not have ADHD or other medical justification for these medications (e.g., narcolepsy), this blood pressure effect and other significant side effects absolutely translate to a net negative impact on health. Nothing in this study or this newsletter should be taken as an argument against the dangers of casual use of amphetamines (e.g., Adderall, Vyvanse) or methylphenidate (e.g., Ritalin) without medical need.
However, this study was designed to answer whether or not, in the context of diagnosed ADHD, use of these medications raises CVD risk to an extent that exceeds the substantial CVD risk associated with unmanaged ADHD. Comparison of baseline characteristics between CVD cases and controls indicates that the former were at higher CVD risk at baseline and may also have experienced more severe ADHD than controls, undermining reported associations with CVD and potentially suggesting that regardless of pharmacological management, more severe ADHD might be associated with a higher CVD risk than more mild cases.
But even if we accept that ADHD medications increase risk of hypertension (based on prior work in addition to this current study), this finding still cannot be taken in isolation as evidence that use of these drugs should be abandoned by all. ADHD itself comes with substantial risks to physical and emotional health, many of which can be mitigated effectively by pharmacological treatment. Risks associated with any drug, supplement, or intervention must be weighed against benefits, and the balance is likely to depend on the individual in question. In light of the rising prevalence of ADHD diagnoses and medication use, more work is needed in order to understand more fully the long-term health implications of the use of various ADHD medications – and how they compare to the considerable long-term implications of unmanaged ADHD.
***
For a list of all previous weekly emails, click here.
***
References
- Zhang L, Li L, Andell P, et al. Attention-Deficit/Hyperactivity Disorder Medications and Long-Term Risk of Cardiovascular Diseases. JAMA Psychiatry. 2024;81(2):178-187. doi:10.1001/jamapsychiatry.2023.4294
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. doi:10.1186/1741-7015-10-99
- Sobanski E, Schredl M, Kettler N, Alm B. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: a controlled polysomnographic study. Sleep. 2008;31(3):375-381. doi:10.1093/sleep/31.3.375
- AlAhmari F, Uddin M. Prevalence of obesity in treated and untreated patients with attention deficit hyperactivity disorder: A meta-analysis. Saudi Med J. 2022;43(8):873-880. doi:10.15537/smj.2022.43.8.20220247
- Raynaud’s Phenomenon. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/raynauds-phenomenon
- Umair HM, Sandler RD, Alunno A, Matucci-Cerinic M, Hughes M. Association between central nervous system stimulants used to treat attention deficit hyperactivity disorder (ADHD) and Raynaud’s phenomenon: A scoping review. Semin Arthritis Rheum. 2021;51(6):1200-1204. doi:10.1016/j.semarthrit.2021.09.002
- Kosheleff AR, Mason O, Jain R, Koch J, Rubin J. Functional Impairments Associated With ADHD in Adulthood and the Impact of Pharmacological Treatment. J Atten Disord. 2023;27(7):669-697. doi:10.1177/10870547231158572




